| Catalogue number | C103864 |
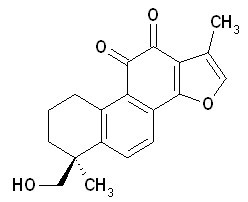
| Chemical name | Tanshinone IIB |
| CAS Number | 17397-93-2 |
| Synonyms | Tanshine IIB;(S)-6-(HydroxyMethyl)-1,6-diMethyl-6,7,8,9-tetrahydrophenanthro[1,2-b]furan-10,11-dione |
| Molecular Weight | C19H18O4 |
| Formula | 310.4 |
| Purity | 98% |
| Physical Description | Red powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Tanshinone IIB (TSB) is a major active constituent of the roots of Salvia miltiorrhiza. Co-treatment with TSB significantly inhibited the cytotoxicity and apoptosis of rat cortical neurons induced by staurosporine in a concentration-dependent manner. Consistently, TSB significantly reduced the DNA laddering caused by staurosporine in a concentration-dependent manner. TSB also suppressed the elevated Bax protein and decreased bcl-2 and caspase-3 proteins induced by staurosporine in rat cortical neurons. These findings indicated that TSB had a neuroprotective effect via inhibition of apoptosis. Further studies are warranted to investigate the role of other apoptosis-related signaling proteins and reperfusion-related mechanisms in the protective effect of TSB on neurons.
|
| References | 1. Phytochemistry, 1991, 30(11), 3815-3817. 2. J. Nat. Prod., 1987, 50(2), 157-160. 3. Phytother Res., 2008, 22(6), 846-850. 4. Neuroscience Letters, 2007, 417(3), 261-265. 5. Journal of Pharmaceutical and Biomedical Analysis, 2007, 44(2), 564-574. 6. Xenobiotica, 2007, 37(4), 375-415. |
| Guestbook |
| C108533 | Cryptochlorogenic acid | $150.00/20mg |
| C104532 | Ginsenoside Rk3 | $238.00/10mg |
| C101167 | Arteannuin B | $230.00/20mg |
| C108063 | 11-Keto-beta-boswellic acid | $134.00/5mg |
| C104424 | Chrysin 6-C-arabinoside 8-C-glucoside | $358.00/5mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com

