| Catalogue number | C101757 |
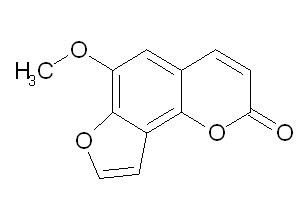
| Chemical name | Sphondin |
| CAS Number | 483-66-9 |
| Synonyms | 6-methoxy-2-furo[2,3-h][1]benzopyranone |
| Molecular Weight | C12H8O4 |
| Formula | 216.2 |
| Purity | 98% |
| Physical Description | Powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Sphondin possessed an inhibitory effect on IL-1beta-induced increase in the level of COX-2 protein and PGE(2) release in A549 cells. Pretreatment of cells with sphondin (10-50 microM) concentration-dependently attenuated IL-1beta-induced COX-2 protein expression and PGE(2) release. The IL-1beta-induced increase in COX-2 mRNA expression was also attenuated by sphondin (50 microM). The selective COX-2 inhibitor, NS-398 (0.01-1 microM), inhibited the activity of the COX-2 enzyme in a concentration-dependent manner, while sphondin (10-50 microM) had no effect. Sphondin (50 microM) did not affect the IL-1beta-induced activations of p44/42 MAPK, p38 MAPK, and JNK. Treatment of cells with sphondin (50 microM) or the NF-kappaB inhibitor, PDTC (50 microM) partially inhibited IL-1beta-induced degradation of IkappaB-alpha in the cytosol and translocation of p65 NF-kappaB from the cytosol to the nucleus. Furthermore, IL-1beta-induced NF-kappaB-specific DNA-protein complex formation in the nucleus was partially inhibited by sphondin (50 microM) or PDTC (50 microM). Taken together, we demonstrate that sphondin inhibits IL-1beta-induced PGE(2) release in A549 cells; this inhibition is mediated by suppressing of COX-2 expression, rather than by inhibiting COX-2 enzyme activity. The inhibitory mechanism of sphondin on IL-1beta-induced COX-2 expression may be, at least in part, through suppression of NF-kappaB activity. We conclude that sphondin may have the therapeutic potential as an anti-inflammatory drug on airway inflammation. |
| References | 1. Acta Pol Pharm., 2003, 60(5), 391-393. 2. Life Sci., 2002, 72(2), 199-213. 3. SQU Journal For Science, 2004, 9, 7-17. |
| Guestbook |
After Receiving
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Featured Products
| C108533 | Cryptochlorogenic acid | $150.00/20mg |
| C108707 | Wogonoside | $80.00/20mg |
| C248429 | Morusin | $158.00/20mg |
| C107861 | Trifolirhizin | $126.00/20mg |
| C257538 | Syringin | $80.00/20mg |
Price(USD)
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders by E-mail
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
Discount Request
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.

