| Catalogue number | C101456 |
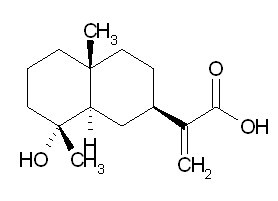
| Chemical name | Ilicic acid |
| CAS Number | 4586-68-9 |
| Synonyms | 2-[(4aR,8R)-8-hydroxy-4a,8-dimethyl-1,2,3,4,5,6,7,8a-octahydronaphthalen-2-yl]-2-propenoic acid |
| Molecular Weight | C15H24O3 |
| Formula | 252.4 |
| Purity | 98% |
| Physical Description | Powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | The present study was designed to examine the anti-inflammatory activity of the sesquiterpenoids ilicic acid and inuviscolide on cell degranulation, leukotriene biosynthesis, neurogenic drive and glucocorticoid-like interactions. Swiss female mice were used to measure the ear oedema induced by phorbol esters or ethyl phenylpropiolate (EPP), and the paw oedema induced by phospholipase A2 (PLA2) or serotonin. Drug treatment consisted of one topically-applied dose in the ear models and a subcutaneous or intraperitoneal injection in the paw models. Quantitative analysis of leukotriene B4 (LTB4) formation was performed on rat peritoneal neutrophils by high performance liquid chromatography (HPLC). The lactone inuviscolide reduced the PLA2-induced oedema (ID50: 98 μmol/kg). The effect on serotonin-induced oedema was not changed by modifiers of the glucocorticoid response. Ilicic acid showed minor in vivo effects, but was slightly more potent than inuviscolide on the 12-O-tetradecanoylphorbol 13-acetate (TPA) acute oedema test (ID50: 0.650 μmol per ear). Inuviscolide reduced LTB4 generation in intact cells, with an IC50 value of 94 μM. On the basis of the reported results, inuviscolide is the main anti-inflammatory sesquiterpenoid from Inula viscosa, and may act by interfering with leukotriene synthesis and PLA2-induced mastocyte release of inflammatory mediators. |
| References | 1. Fitoterapia, 2003, 74(5), 459-463. 2. Planta Med., 2001, 67(8), 726-731. 3. Acta Botanica Yunnanica, 1997, 19(2), 207-210. |
| Guestbook |
After Receiving
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Featured Products
| C104532 | Ginsenoside Rk3 | $238.00/10mg |
| C104615 | Kushenol C | $468.00/5mg |
| C104426 | Chrysin 6-C-glucoside 8-C-arabinoside | $358.00/5mg |
| C107991 | Salvianolic acid B | $64.00/20mg |
| C262180 | Verbascoside | $72.00/20mg |
Price(USD)
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders by E-mail
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
Discount Request
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.

