| Catalogue number | C100196 |
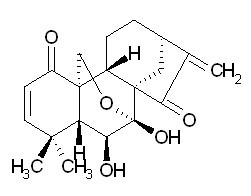
| Chemical name | Eriocalyxin B |
| CAS Number | 84745-95-9 |
| Synonyms | Kaura-2,16-diene-1,15-dione,7,20-epoxy-6,7- dihydroxy-,(6a,7R)-;Rabdosianone I; |
| Molecular Weight | C20H24O5 |
| Formula | 344.4 |
| Purity | 98% |
| Physical Description | Cryst. |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | A potent NF-kappaB inhibitor, eriocalyxin B (Eri-B), an ent-kauranoid isolated from Isodon eriocalyx, an anti-inflammatory remedy. The presence of two alpha, beta-unsaturated ketones give this compound the uniqueness among the ent-kauranoids tested. Eri-B inhibited the NF-kappaB transcriptional activity but not that of cAMP response element-binding protein. It suppressed the transcription of NF-kappaB downstream gene products including cyclooxygenase-2 and inducible nitric-oxide synthase induced by tumor necrosis factor-alpha or lipopolysaccharide in macrophages and hepatocarcinoma cells. Chromatin immunoprecipitation assay indicated that Eri-B selectively blocked the binding between NF-kappaB and the response elements in vivo without affecting the nuclear translocation of the transcription factor. Down-regulation of the endogenous p65 protein sensitized the cells toward the action of the compound. Furthermore, in vitro binding assays suggested that Eri-B reversibly interfered with the binding of p65 and p50 subunits to the DNA in a noncompetitive manner. In summary, this study reveals the novel action of a potent NF-kappaB inhibitor that could be potentially used for the treatment of a variety of NF-kappaB-associated diseases. Modification of the structure of this class of compounds becomes the key to the control of the behavior of the compound against different cellular signaling pathways.
|
| References | 1. Acta Botanica Yunnanica, 2000, 22(3), 337-342. 2. Mol Pharmacol., 2006, 70(6), 1946-1955. 3. Cell death and differentiation, 2007, 14(2), 306-317. |
| Guestbook |
| C108063 | 11-Keto-beta-boswellic acid | $134.00/5mg |
| C104615 | Kushenol C | $468.00/5mg |
| C108812 | Ginsenoside Compound K | $126.00/20mg |
| C104063 | Quercetin-3-O-glucuronide | $286.00/10mg |
| C104532 | Ginsenoside Rk3 | $238.00/10mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com

