| Catalogue number |
C101241 |
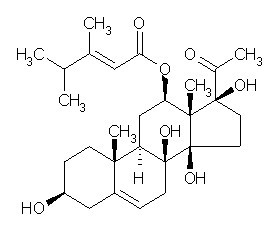
| Chemical name | Caudatin |
| CAS Number | 38395-02-7 |
| Synonyms | (3beta,12beta,14beta,17alpha)-12-[[(2E)-3,4-Dimethyl-1-oxo-2-pentenyl]oxy]-3,8,14,17-tetrahydroxypregn-5-en-20-one |
| Molecular Weight | C28H42O7 |
| Formula | 490.6 |
| Purity | 98% |
| Physical Description | Powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Caudatin exhibited significantly inhibitory activity against HBV DNA replication with IC50 values in the range of 2.82-7.48 μM.
Caudatin exerts antiproliferative effects on human hepatocellular carcinoma SMMC7721 cells. The anticancer activity of caudatin could be attributed partly to its inhibition of cell proliferation and induction of apoptosis in cancer cells through caspase activation. Then the in vivo assay further showed that caudatin significantly inhibited the growth of transplantable H22 tumors in mice.
caudatin impairs the cell viability and induces G0/G1 phase arrest in A549 cells with a dose dependent manner. A549 cells, not HUVECs, dealing with caudatin exhibited typical characteristics of apoptosis, which were accompanied by activation of caspase-3, caspase-9 and Poly(ADP–Ribose) Polymerase (PARP). In addition, caudatin treatment resulted in a decrease of β-catenin and increase of phosphorylation of β-catenin, and inhibited phosphorylation levels of GSK3β (Ser 9) in A549 cells. Conditional medium of A549 cells-induced or growth factors-induced tube formation of HUVECs was markedly inhibited by caudatin treatment, which was associated with the inhibiting VEGF secretion from A549 cells by caudatin. Our findings suggest that caudatin inhibits carcinomic human alveolar basal epithelial cell growth and angiogenesis by targeting GSK3β/β-catenin pathway and suppressing VEGF production.
|
| References | 1. Letters in Drug Design & Discovery, 2012, 9(8), 775-779.
2. Steroids, 2007, 72(11-12), 778-786.
3. Chinese Journal of Natural Medicines, 2008, 6(3), 210-213.
4. Phytomedicine, 2008, 15(11), 1016-1020.
5. J. Cell. Biochem., 2012, 113, 3403-3410.
|
| Guestbook |
|
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.