| Catalogue number |
C107880 |
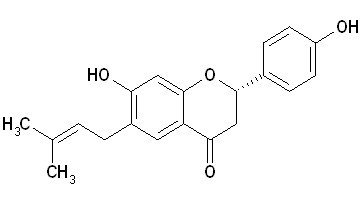
| Chemical name | Bavachin |
| CAS Number | 19879-32-4 |
| Synonyms | 4H-1-benzopyran-4-one, 2,3-dihydro-7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methyl-2-buten-1-yl)-;7-Hydroxy-2-(4-hydroxyphenyl)-6-(3-methylbut-2-en-1-yl)-2,3-dihydro-4H-chromen-4-one; Coryfolin |
| Molecular Weight | C20H20O4 |
| Formula | 324.4 |
| Purity | 98% |
| Physical Description | Powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Bavachin is a weak antioxidant. Antimutagenic.
Bavachin stimulates bone formation and has potential activity against osteoporosis. Shows inhibitory activities against the antigen-induced degranulation and weak estrogen-like activity.
Bavachin could potently decrease IL-1 beta-induced nuclear factor-kappa B (NF-kappaB) but not activator protein-1 (AP-1) DNA-binding activity. Bavachin also inhibited I kappaB alpha degradation, increased nuclear translocation of p65 and p50 as well as decreased I kappaB alpha kinase (IKK) activity. Furthermore, bavachin inhibited IL-1 beta-induced chemokine production that resulted in reduced migration of THP-1 monocytic cells. Through decreasing IL-1 beta-induced activation of IKK-I kappaB alpha-NF-kappaB signaling pathway, bavachin potentially protects cartilage from inflammation-mediated damage in joints of osteoarthritis patients.
The IC50 values were 86.0 μM in the Acyl-coenzyme A: cholesterol acyltransferase (ACAT) assay system using rat liver microsome. ACAT catalyzes cholesterol esterification and plays important roles in intestinal absorption of cholesterol, hepatic production of lipoproteins and accumulation of cholesteryl ester within macrophages and smooth muscle cells.
Bavachin characterized its estrogenic activity by ligand binding, reporter gene activation, and endogenous estrogen receptor (ER) target gene regulation. Bavachin showed ER ligand binding activity in competitive displacement of [3H] E2 from recombinant ER. The estrogenic activity of bavachin was characterized in a transient transfection system using ER or ER and estrogen-responsive luciferase plasmids in CV-1 cells with an EC50 of 320 nM and 680 nM, respectively. Bavachin increased the mRNA levels of estrogen-responsive genes such as pS2 and PR, and decreased the protein level of ER by proteasomal pathway. However, bavachin failed to activate the androgen receptor in CV-1 cells transiently transfected with the corresponding receptor and hormone responsive reporter plasmid. These data indicate that bavachin acts as a weak phytoestrogen by binding and activating the ER.
|
| References | 1. Biol. Pharm. Bull., 2005, 28(12), 2253-2257
2. European Journal of Pharmacology, 2010, 636(1-3), 181-188.
3. Archives of Pharmacal Research, 2008, 31(11), 1419-1423.
|
| Guestbook |
|
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.