| Catalogue number |
C107947 |
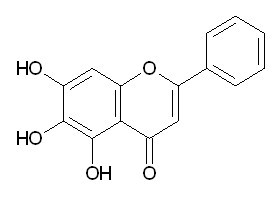
| Chemical name | Baicalein |
| CAS Number | 491-67-8 |
| Synonyms | 5,6,7-trihydroxy-2-phenyl-1-benzopyran-4-one |
| Molecular Weight | C15H10O5 |
| Formula | 270.2 |
| Purity | 98% |
| Physical Description | Yellow powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Baicalein has been shown to inhibit certain types of lipoxygenases and act as an anti-inflammatory agent. It has antiproliferative effects on ET-1-induced proliferation of pulmonary artery smooth muscle cell proliferation via inhibition of TRPC1 channel expression. Possible antidepressant effects have also been attributed to baicalein in animal research.
Baicalein and baicalin demonstrated a strong activity on eliminating the superoxide radical (O2-) (baicalein: 7.31 x 10(4) u/g; baicalin: 1.19 x 10(5) u/g). The IC50 of baicalein was 2.8 fold higher than that of baicalin. However they had no significant effect on scavenging hydroxyl radical (OH). The present results demonstrated that baicalein and baicalin posed a different pathological pathway. The antioxidant function of baicalin was mainly based on scavenging superoxide radical whilst baicalein was a good xanthine oxidase inhibitor.
Baicalein is an inhibitor of CYP2C9, an enzyme of the cytochrome P450 system that metabolizes drugs in the body.
In vitro the radical scavenging effect of baicalein in detail using electron spin resonance spectrometry. Furthermore, we examined in vivo its effect on the thiobarbituric acid-reactive substances (TBARS) levels and superoxide dismutase in the brain of rats with FeCl3-induced epilepsy and on hippocampal delayed neuronal death in gerbils with transient ischemia. In in vitro experiments, baicalein quenched in a dose-dependent manner 1,1-diphenyl-2-picrylhydrazyl, superoxide, and hydroxyl radicals. In the FeCl3-induced epileptic model, baicalein suppressed the increase in the TBARS level at the FeCl3-injected site. Baicalein also inhibited hippocampal neuronal death induced by 5 min of cerebral ischemia in gerbils. Hence the present study suggested that baicalein is one of the active components in TJ-960, which partially contributes to the antiepileptic and neuronal protective effects of TJ-960, and that the mechanism of its pharmacological action is based upon radical quenching and antioxidative effects.
Low micromolar concentrations of baicalein, and especially its oxidized forms, inhibit the formation of α-synuclein fibrils. In addition, existing fibrils of α-synuclein are disaggregated by baicalein. The product of the inhibition reaction is predominantly a soluble oligomer of α-synuclein, in which the protein molecules have been covalently modified by baicalein quinone to form a Schiff base with a lysine side chain in α-synuclein. The binding of baicalein was abolished by conversion of the Tyr residues into Phe, demonstrating that Tyr is involved in the interaction of α-synuclein with baicalein. In disaggregation baicalein causes fragmentation throughout the length of the fibril. These observations suggest that baicalein and similar compounds may have potential as therapeutic leads in combating Parkinson's disease and that diets rich in flavonoids may be effective in preventing the disorder.
|
| References | 1. Mol. Cells, 2001, 12(1), 127-130.
2. Magnetic Resonance in Chemistry, 2008, 46(3): 215-225.
3. Journal of Pharmacy and Pharmacology, 2005, 57(2), 219-225.
4. Anticancer Research, 2000, 20(5A), 2861-2865.
5. Archives of Biochemistry and Biophysics, 1993, 306(1), 261-266.
|
| Guestbook |
|
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.