| Catalogue number |
C100747 |
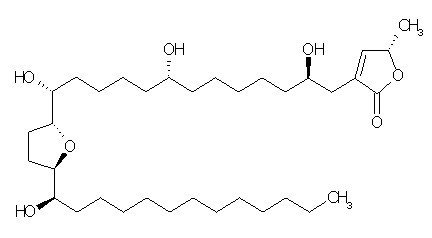
| Chemical name | Annonacin |
| CAS Number | 111035-65-5 |
| Synonyms | 4-[2,8-dihydroxy-12-[[5-(1-hydroxytridecyl)-2-oxolanyl]methoxy]dodecyl]-2-methyl-2H-furan-5-one |
| Molecular Weight | C35H64O7 |
| Formula | 596.8 |
| Purity | 98% |
| Physical Description | Powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Annonacin is highly cytotoxic and is active in an assay designed to detect antimitotic agents.
Annonacin inhibited complex I in brain homogenates in a concentration-dependent manner, and, when administered systemically, entered the brain parenchyma, where it was detected by matrix-associated laser desorption ionization – time of flight mass spectrometry, and decreased brain ATP levels by 44%. In the absence of evident systemic toxicity, we observed neuropathological abnormalities in the basal ganglia and brainstem nuclei. Stereological cell counts showed significant loss of dopaminergic neurones in the substantia nigra (−31.7%), and cholinergic (−37.9%) and dopamine and cyclic AMP-regulated phosphoprotein (DARPP-32)-immunoreactive GABAergic neurones (−39.3%) in the striatum, accompanied by a significant increase in the number of astrocytes (35.4%) and microglial cells (73.4%). The distribution of the lesions was similar to that in patients with atypical parkinsonism. These data are compatible with the theory that annonaceous acetogenins, such as annonacin, might be implicated in the aetiology of Guadeloupean parkinsonism and support the hypothesis that some forms of parkinsonism might be induced by environmental toxins.
Annonacin, a natural mitochondrial Complex I inhibitor, causes Tau pathology in cultured neurons. The annonacin-induced ATP depletion causes the retrograde transport of mitochondria to the cell soma and induces changes in the intracellular distribution of tau in a way that shares characteristics with some neurodegenerative diseases.
Annonacin caused significant cell death in various cancer cell lines. T24 bladder cancer cells at the S phase were more vulnerable to the cytotoxicity of annonacin. Furthermore, annonacin activated p21 in a p53-independent manner and arrested T24 cells at the G1 phase. It also induced Bax expression, enhanced caspase-3 activity, and caused apoptotic cell death in T24 cells. In summary, these results suggest that annonacin is potentially a promising anti-cancer compound.
|
| References | 1. Experientia, 1987, 43(8), 947-949.
2. Journal of Neurochemistry, 2004, 88(1), 63-69.
3. Life Sciences, 2003, 72(25), 2853-2861.
4. The Journal of Neuroscience, 2007, 27(29), 7827-7837.
|
| Guestbook |
|
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.